Technology
NK Cells
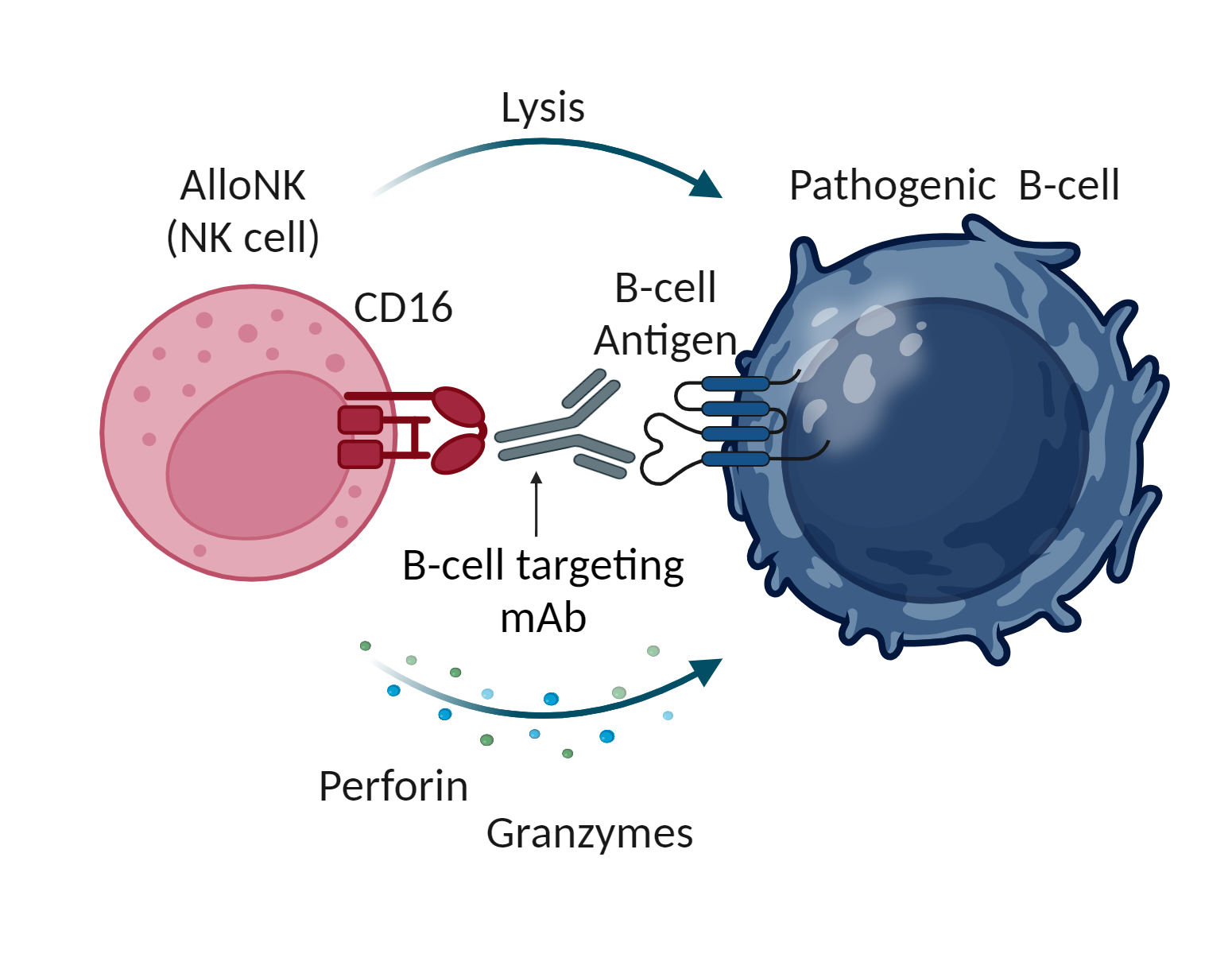
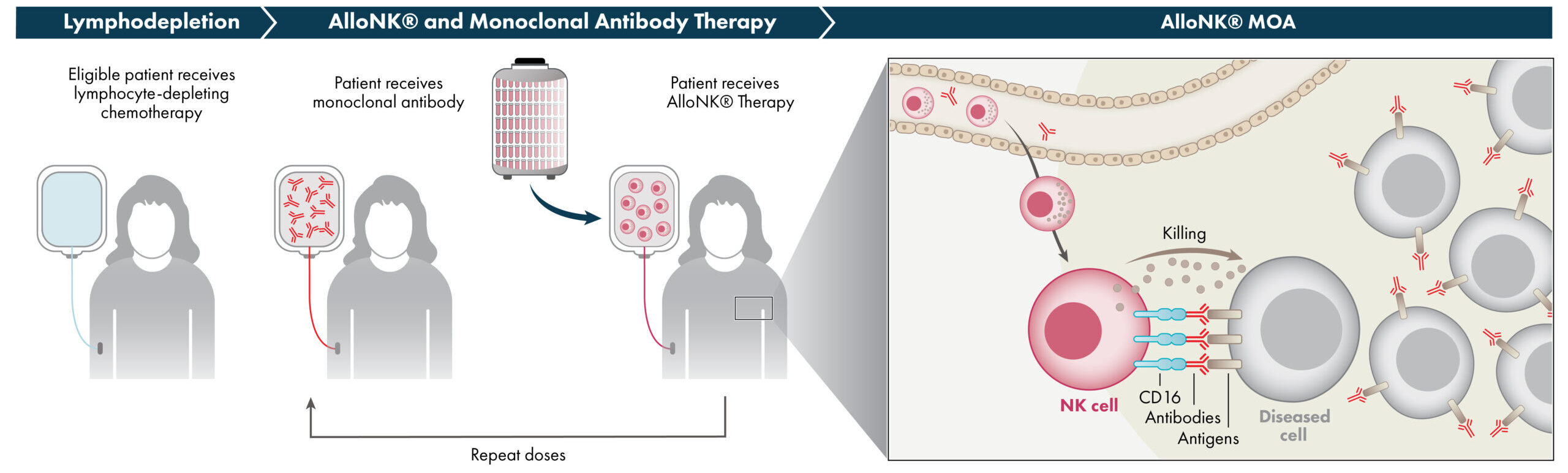
NK cells are part of the innate immune system, which is the body’s first line of immune surveillance and defense. NK cells modulate their activity through a balance of activating and inhibiting receptors on their surface that engage with their environment including with target cells. This balance enables NK cells to recognize and kill abnormal cells while suppressing cytotoxic responses to normal tissue, thereby providing a population of immune effector cells that defend against cancer and virus-infected cells. NK cells naturally work in concert with antibodies. The antibody first binds to a target on a diseased cell, then to the NK cell via the cell-surface receptor CD16, which engages the antibody to mount an ADCC response. NK cells may have an advantage over other immune cells, such as the T-cells used in CAR-T cell therapy and other cell therapies, because they can be used as allogeneic therapies, meaning that NK cells from one donor can be administered to one or many patients without the requirement for gene editing or other genetic manipulations.
AlloNK®
AlloNK is an allogeneic, off-the-shelf, cryopreserved NK cell therapy candidate designed to enhance the ADCC effect of mAbs to drive B-cell depletion. AlloNK, as a non-genetically modified, non-targeted NK cell, is designed to utilize a mAb or NK engager for targeting, and therefore can be used in different combinations against different therapeutic targets. AlloNK is currently in clinical trials for treatment of systemic lupus erythematosus (SLE) for patients with or without lupus nephritis and in a basket investigator-initiated trial (IIT) in multiple autoimmune indications. In addition, AlloNK is being evaluated for the treatment of non-Hodgkin lymphoma, as well as in combination with Affimed’s innate cell engager acimtamig for the treatment of patients with relapsed/refractory CD30-positive lymphomas.
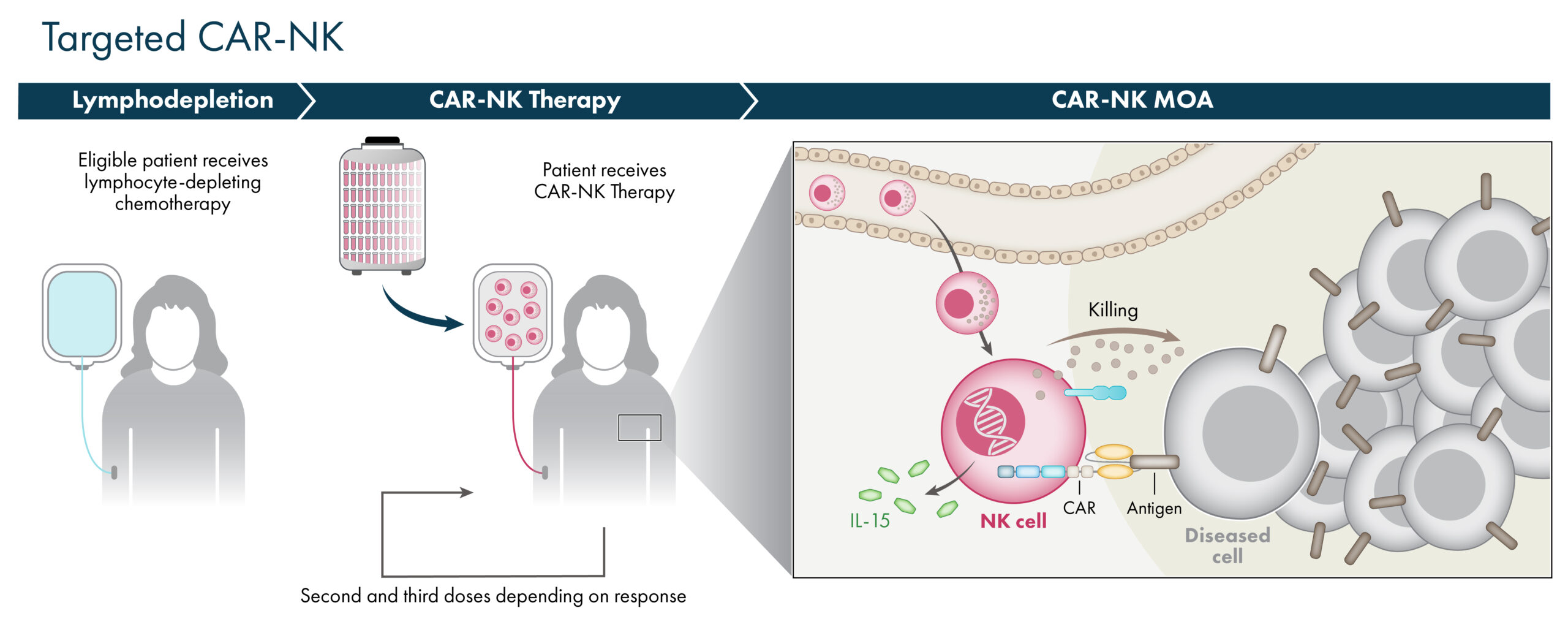
Targeted CAR-NK Cells
As an alternative to targeting NK cells by combining with mAbs or NK engagers, NK cells may be targeted directly through the addition of a chimeric antigen receptor (CAR) against a defined target antigen. This is accomplished through genetic modification of the NK cell and can also include the addition of cytokine transgenes to further enhance the activity and persistence of these CAR-NK cells. Our manufacturing platform allows for the generation of CAR-targeted NK cells utilizing cord blood starting material with the same pre-selected characteristics and scale-up process as AlloNK.
Manufacturing-First Approach
Artiva’s platform generates allogeneic natural killer (NK) cells from healthy donor umbilical cord blood (UCB) units selected for the high affinity variant of the CD16 receptor (158 V/V) and a KIR-B haplotype, which are both markers that have been shown in preclinical studies and published clinical trials to increase the activity of NK cells. Product candidates derived from our manufacturing process contain these genetic attributes without need for genetic modification. Our manufacturing process leverages over a decade’s work by our strategic partner, GC Cell Corporation (GC Cell). Today, with the manufacturing facility in our San Diego headquarters we have the capability to produce enough AlloNK to treat over 1,000 autoimmunity patients annually. Our process is designed to allow us to produce off-the-shelf, allogeneic NK cell therapy candidates and to potentially meet the scale of commercial demand, with the mission to make these therapies broadly accessible for patients with devastating autoimmune diseases and cancers.
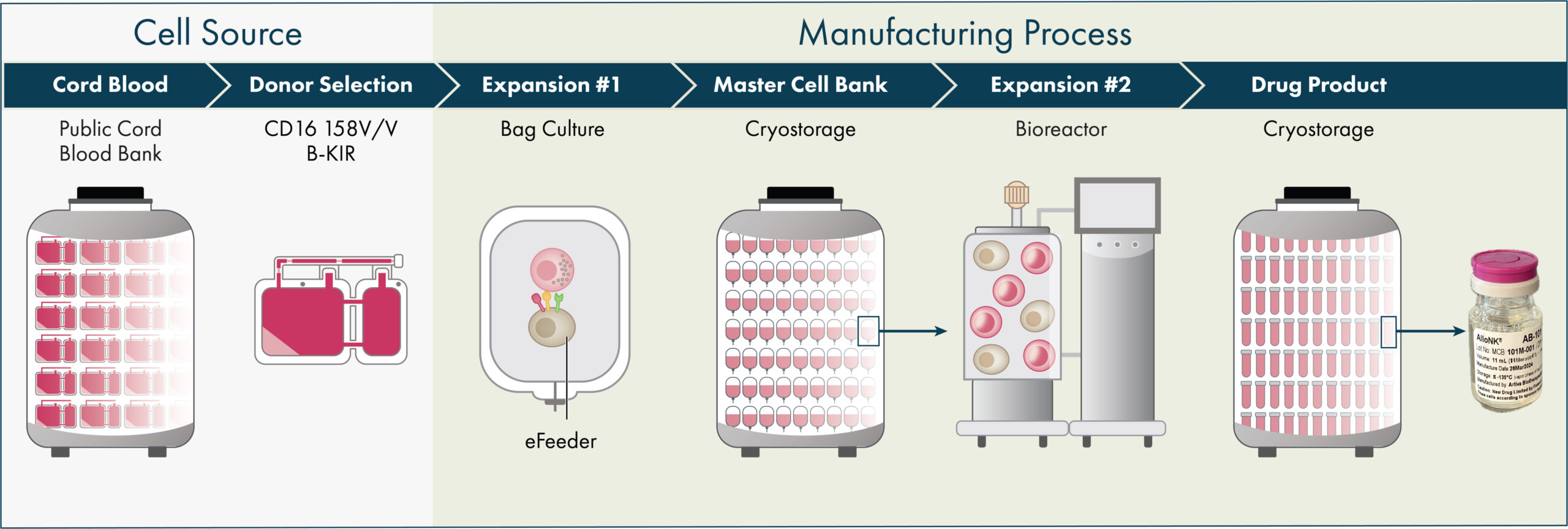
Cord blood units with preferred characteristics, including the high affinity variant of CD16 known as 158 V/V and the KIR-B haplotype, are pre-selected from cord blood banks. Approximately 15% of available cord blood units have these characteristics. NK cells from cord blood units are isolated, expanded and activated using our proprietary feeder cell process then purified and cryopreserved creating a master cell bank (MCB). Our platform allows for the introduction of an engineered vector for our CAR-NK programs at this step in the process. At the current scale, each cord blood unit is expanded to yield 50-80 cryopreserved MCB units. In turn, each MCB unit is further expanded to yield 80-100+ one billion-cell drug product vials. Drug product is vialled in an infusion-ready media and cryopreserved, providing long-term stability and ease of handling at clinical sites. To date, we and GC Cell have produced over 35 clinical batches of AlloNK, producing thousands of AlloNK vials at one billion cells per vial, and have demonstrated both batch-to-batch and donor-to-donor consistency.
Artiva’s San Diego corporate headquarters and R&D labs are based in a 52,000-square-foot facility that includes a 9,000 square foot, multi-suite, purpose-built current Good Manufacturing Practices (cGMP) cell production center, to support NK and CAR-NK cell production for our pipeline development, clinical trial, and potentially commercial supply. At the current 50-liter scale, this facility has the potential to produce over 6,000 one billion-cell vials annually. We are currently developing a 200-liter, commercial-scale process that has the potential to efficiently supply many thousands of patients with a cost of goods below $1,000 per one billion-cell vial.

Artiva has a purpose-built research, process development, and multi-suite NK manufacturing center in San Diego, California.


Artiva also has an exclusive partnership with GC Cell and access to state-of-the-art cell therapy research and cGMP manufacturing in Seoul, Korea.