Technology
NK Cells
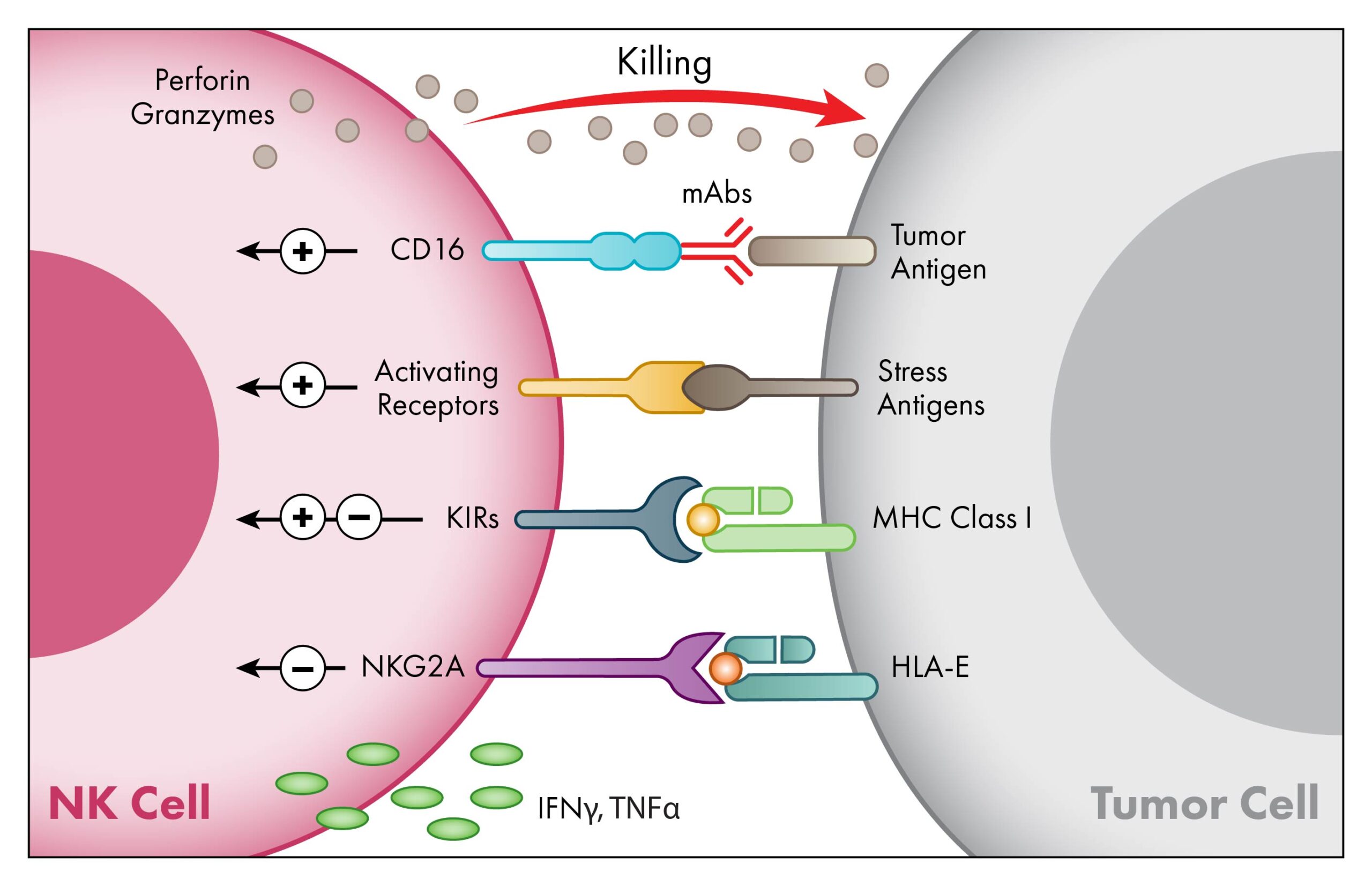
NK cells are the body’s first line of defense against tumor or other pathogenic cells. Upon activation, NK cells release perforins and granzymes to kill the target cells and secrete cytokines to recruit and engage other immune cell’s activity. NK cells are the natural mediators of antibody-dependent cellular cytotoxicity (ADCC). Monoclonal antibody or NK-engager protein therapies interact with CD16 on the surface of NK cells, activating them to kill targeted cells. Unlike T-cells, NK cells have a reduced risk for alloreactivity and graft-versus-host disease (GvHD).
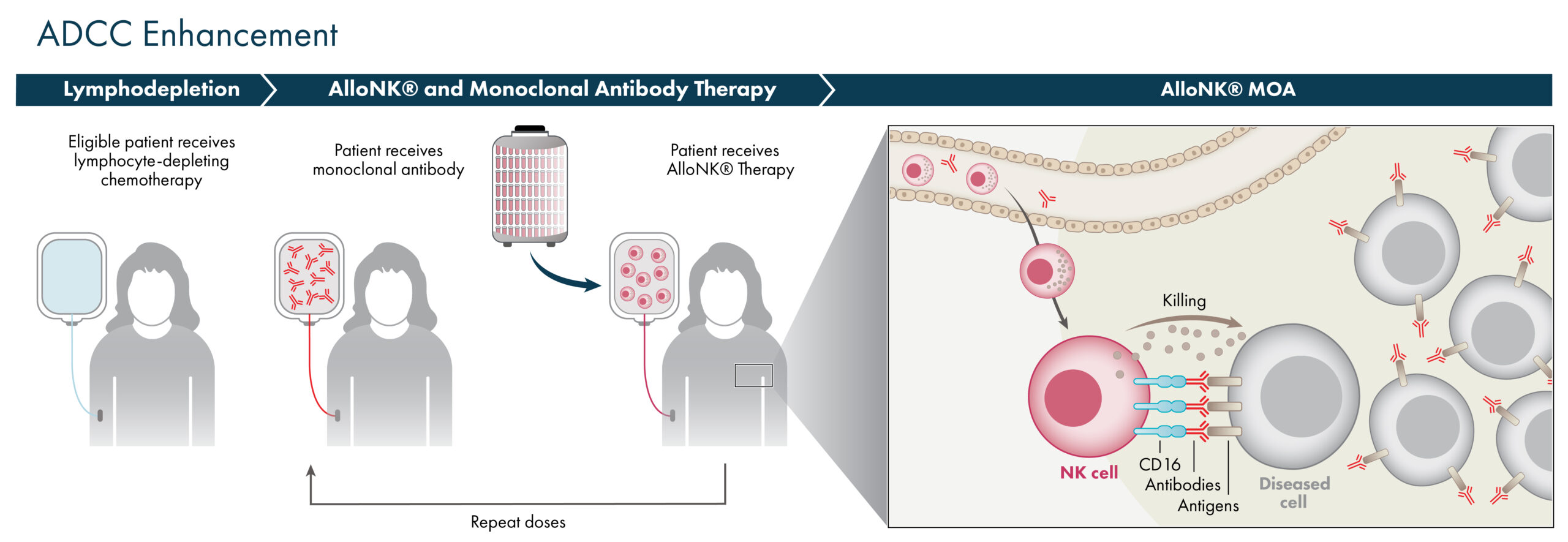
AlloNK® Non-Genetically Modified ADCC Enhancer
AlloNK® (also known as AB-101) is an allogeneic, non-genetically modified NK cell therapy candidate designed to enhance the antibody-dependent cellular cytotoxicity (ADCC) effect of monoclonal antibodies or NK cell engagers. Artiva selects cord blood units with the high affinity variant of the CD16 receptor and a KIR-B haplotype to increase the activity of a targeting antibody or NK cell engager. AlloNK can enhance the ADCC effect of a targeting monoclonal antibody or NK cell engager to elicit the elimination of pathogenic autoantibody producing B-cells or cancerous cells. AlloNK can be combined with different antibodies or NK cell engagers to attack different targets on pathogenic cells.
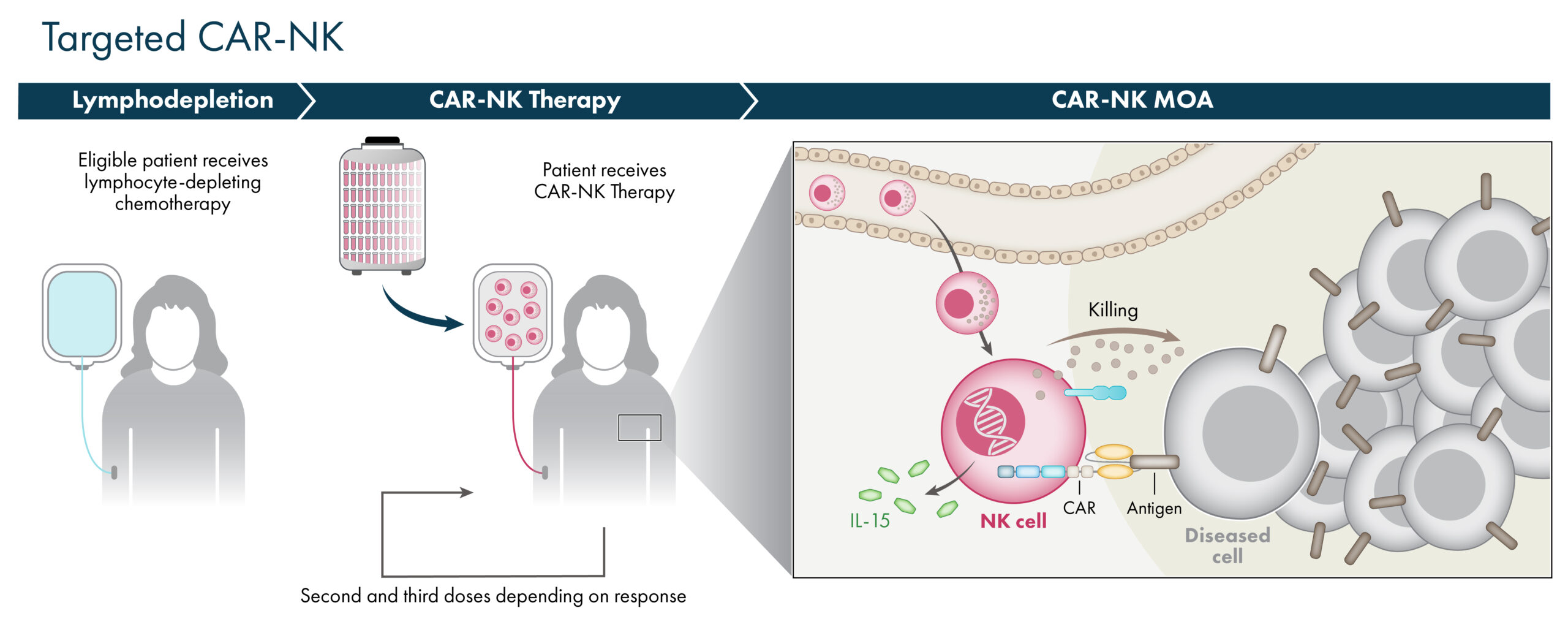
Targeted CAR-NK Cells
Artiva’s NK cells, engineered to express chimeric antigen receptors, or CARs, have the potential to enhance the targeting and activity of the NK cells against either hematologic or solid tumors. Artiva utilizes a proprietary CAR platform optimized for use in NK cells to increase their therapeutic activity and tumor targeting capability. Additionally, all CAR-NK products are manufactured via Artiva’s cell therapy manufacturing platform and maintain high expression of CD16, enabling dual targeting therapeutic approaches via monoclonal antibody combinations.
Cell Therapy Manufacturing Platform and Infrastructure
Artiva’s platform generates allogeneic natural killer (NK) cells from healthy donor umbilical cord blood (UCB) units selected for key characteristics. Our Manufacturing-First approach has created a highly scaled process capable of producing enough consistently active NK cells from each UCB to treat hundreds to thousands of patients, with the opportunity for repeat dosing in the outpatient setting. Our NK cells are cryopreserved and ready for off-the-shelf therapeutic use at the clinical site.
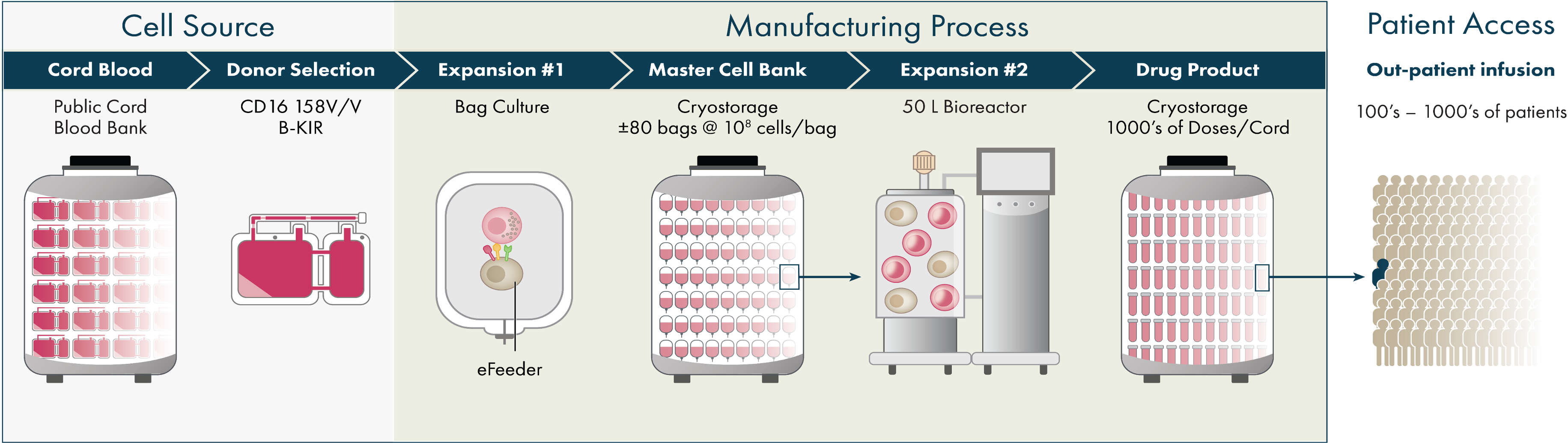
Artiva’s proprietary cell therapy manufacturing platform utilizes cell expansion and activation technology developed and improved for over a decade by our strategic partner, GC Cell. Umbilical cord blood (UCB) units with preferred characteristics, including the high affinity variant of CD16 (158 V/V) and the B-KIR haplotype, are selected from registered cord blood banks. Each UCB unit is used to derive a NK cell master cell bank, followed by a bioreactor-based large-scale NK cell expansion and activation process to produce highly active NK cells. The scale of production has the potential to enable hundreds to thousands of patients to be treated from a single donor UCB unit with a cost of production that we believe will support wider adoption of a cell therapy product. Optimized cryopreservation of the NK cells in infusion-ready media and demonstrated long-term stability, cold chain logistics, and ease of handling at the clinical site enable repeat dosing in the outpatient setting.
Artiva Biotherapeutics is advancing a pipeline of highly scaled, cryopreserved, off-the-shelf, allogeneic NK cell therapies for treating cancers and immune disorders. Our two-fold product strategy aims to harness the innate biology of NK cells with the intent of maximizing their therapeutic potential.
Artiva’s San Diego corporate headquarters and R&D labs are based in a 52,000-square-foot facility that includes a multi-suite,purpose-built current Good Manufacturing Practices (cGMP) cell production center, to support NK and CAR-NK cell production for Artiva’s pipeline development and clinical trial supply. GC Cell has invested significantly in state-of-the-art research and GMP manufacturing for the development of cell therapies. Artiva and GC Cell have established an exclusive partnership to further realize the clinical application of NK cell therapy.

Artiva has a purpose-built research, process development, and multi-suite NK manufacturing center in San Diego, California.


Artiva also has an exclusive partnership with GC Cell and access to state-of-the-art cell therapy research and cGMP manufacturing in Seoul, Korea.